ChemInform Abstract: Conformationally Constrained Analogues of Diacylglycerol. Part 12. Ultrapotent Protein Kinase C Ligands Based on a Chiral 4,4- Disubstituted Heptono-1,4-lactone Template.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
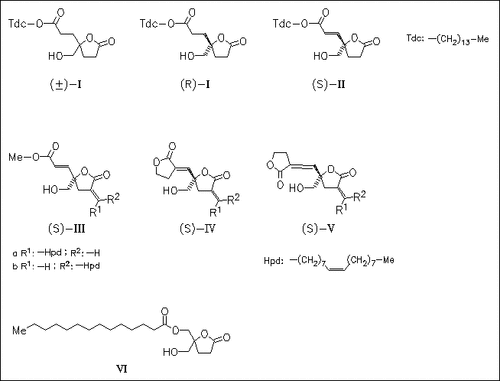
Conformationally constrained analogues of diacylglycerol (DAG) built on a 5-((acyloxy)methyl)-5-(hydroxymethyl)tetrahydro-2-furanone such as (VI) are shown previously to bind tightly to protein kinase Cα (PK-Cα) in a stereospecific manner. These compounds, however, racemize readily through rapid acyl migration and lose biological potency. In order to circumvent this problem, the “reversed ester” analogues (I)-(V) are designed as a new set of PK-C ligands. The reversed ester analogues are impervious to racemization, and their chemically distinct branches facilitate the enantiospecific syntheses of all targets. Conversion of the propanoyl branch into a propenoyl branch and functionalization of the propenoyl-branched compounds as . alpha.-alkylidene lactones produce stable compounds with equiv. ultrapotent binding affinities for PK-C ((II), (III), (IV)). The biological activity of the most potent agonist (IIIb) is evaluated further.