ChemInform Abstract: Inversion of Stereochemistry in the Co2(CO)8-Catalyzed Carbonylation of Aziridines to β-Lactams. First Synthesis of Highly Strained trans-Bicyclic β-Lactams.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
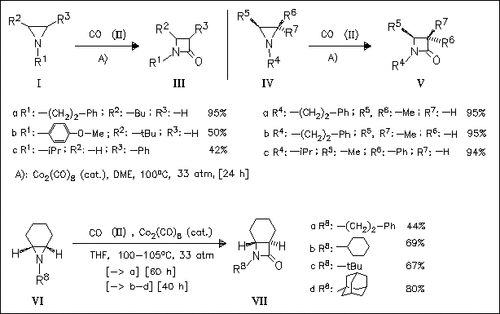
β-Lactams such as (III) and (V) are synthesized by the carbonylative ring expansion of aziridines catalyzed by Co2(CO)8 under CO pressure. The active catalyst (Co(CO)4)- induces nucleophilic ring opening of the heterocycle resulting in inversion of configuration (( IV) → (V)). The regio- and stereospecificity of this reaction results in the synthesis of the first highly strained trans-7- azabicyclo(4.2.0)octan-8-one derivatives such as (VII).