ChemInform Abstract: (E)-(Arylmethyleneaminoxy)acetamides as Analogues of Neuroleptic Benzamides: Synthesis and D2-Dopaminergic Binding Affinity.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
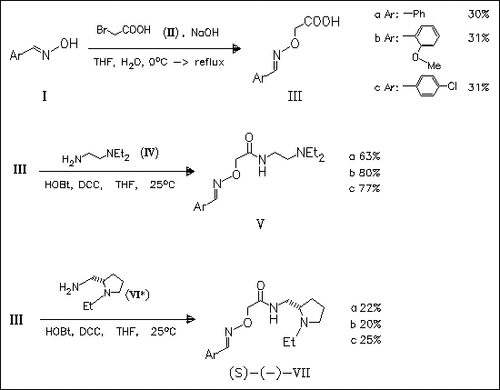
Analogues of the neuroleptic benzamide metoclopramide and (S)- remoxipride, in which the aromatic group is substituted by a ( methyleneaminooxy)methyl moiety (cf. (V) and (VII), resp.), are synthesized and evaluated for their D2-dopaminergic binding affinity. Biological studies indicate that the (methyleneaminooxy)methyl moiety cannot be considered as a bioisoster of the aryl in neuroleptic benzamides.