ChemInform Abstract: Catalytic Triple Carbonylation of Olefins. Enantioselective Synthesis of 2-Oxoglutarates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
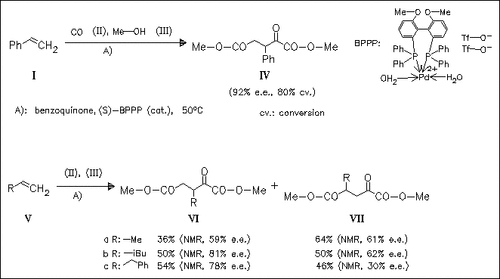
The new title reaction allows a one-step synthesis of optically active substituted 2-oxoglutarates with fair to excellent enantioselectivities. The reaction is completely regioselective for styrene, whereas with aliphatic olefins 2 regioisomers (VI) and (VII) are formed in ratios of ≅1:2 and ≅1:1, respectively.