ChemInform Abstract: A Highly Diastereoselective and Enantioselective Ti(OTos)2-TADDOLate- Catalyzed 1,3-Dipolar Cycloaddition Reaction of Alkenes with Nitrones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
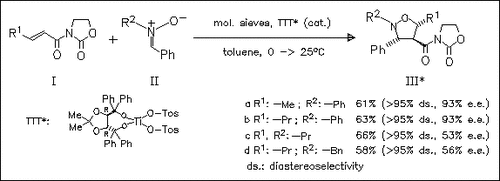
It is shown that the tosylate groups at the Ti atom of TTT is of utmost importance for both diastereo- and enantioselectivities of the title reaction. endo-Isoxazolidines, e.g. (IIIa) and (IIIb), with high diastereo- and enantioselectivity are obtained from N-phenyl nitrones. Reactions of N-alkyl and N-benzyl nitrones, e.g. (IIc) and (IId) give the endoisoxazolidines (IIIc) and (IIId) with the same diastereoselectivity, but the e.e. is in the range of 40-56%.