ChemInform Abstract: Total Syntheses of (+)-Acutiphycin (Ia) and (+)-trans-20,21- Didehydroacutiphycin (Ib).
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
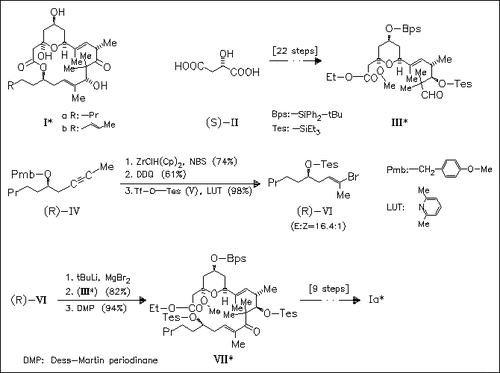
The macrolides (I) of significant in vivo antineoplastic activity and cytotoxicity are prepared similarly. A key step is represented by the highly stereoselective hydrozirconation-bromination of (IV).