ChemInform Abstract: Diastereoselective Intramolecular Cyclization Through the Triphenylphosphine/Carbon Tetrachloride System: Synthesis of Saturated 1,4-Dihetero Seven-Membered Cycloacetals.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
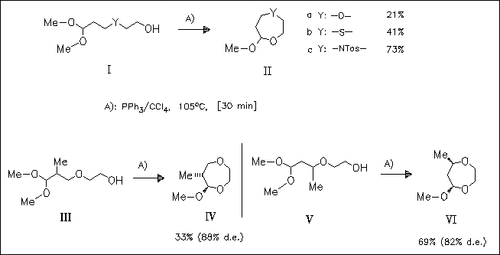
Treatment of various 4-hetero-6-hydroxyalkanal dimethyl acetals (I) with the triphenylphosphine/carbon tetrachloride system results in cyclization to the corresponding 1,4-heteroxepane derivatives (II). The high diastereoselectivity of the cyclizations of the methyl- substituted educts (III) and (V) gives evidence for the reported ionic cyclization mechanism.