ChemInform Abstract: Cyclization of a Chiral N-Crotyl Methoxycarbonylacetamide Mediated by Mn(III). An Easy Entry to (R)-3-Pyrrolidineacetic Acid.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
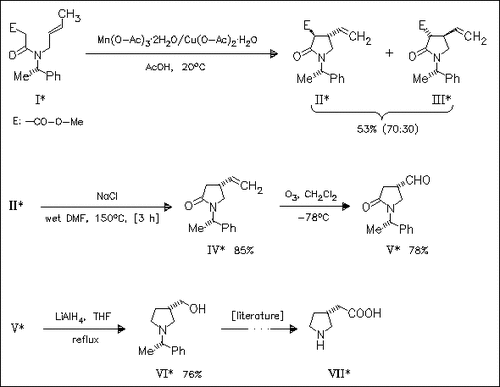
As part of a program aimed at preparing non-proteinogenic amino acids, the synthesis of diastereomerically pure 4-substituted pyrrolidin-2- ones and their conversion to 3-substituted pyrrolidones is studied. Thus, Mn(III)-mediated diastereoselective cyclization of the amide (I) gives an easy separable mixture of the diastereomeric pyrrolidin-2- ones (II) and (III) of which the major isomer (II) is a key intermediate to (R)-3-pyrrolidineacetic acid (VII), an inhibitor of GABA uptake in astrocytes.