ChemInform Abstract: Diastereoselective Reduction of α-(p-Tolylsulfinyl)ketoximes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
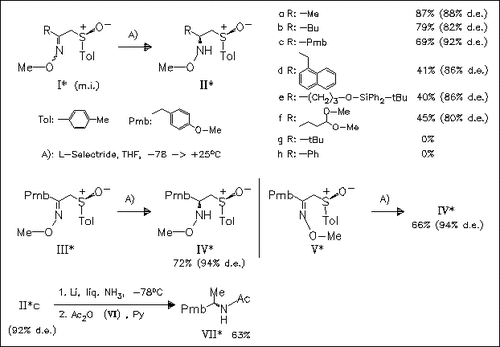
Treatment of α-sulfinylketoximes according to A) results in the first reported diastereoselective reduction via a nonchelate model yielding (S)-(N-methoxyamino)sulfoxides. Further reductive fission of the C-S and N-O bonds of the products, e.g. (IIc) followed by acetylation leads to chiral amines (V), thus providing a suitable approach to this type of compounds.