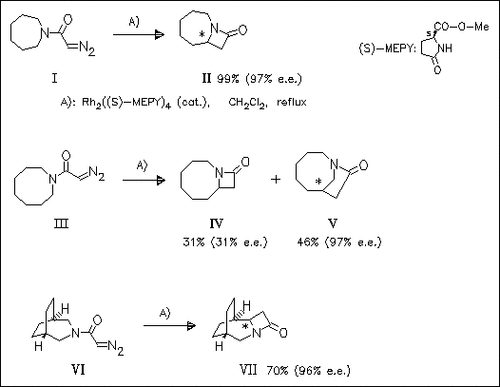
ChemInform Abstract: Highly Enantioselective Route to β-Lactams via Intramolecular C-H Insertion Reactions of Diazoacetylazacycloalkanes Catalyzed by Chiral Dirhodium(II) Carboxamidates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The carbenes obtained by decomposition of the diazoacetylazacycloalkanes (I), (III), and (VI) undergo highly enantioselective intramolecular C-H insertion reactions under conditions A) to form the corresponding β- and γ-lactams.