ChemInform Abstract: Lipase-Mediated Resolution of Inden-1-ol.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
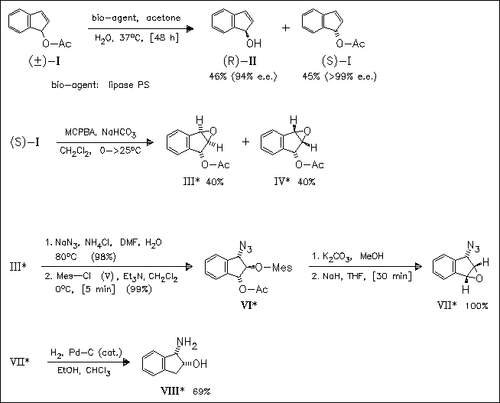
All attempts of a kinetic acetylation of racemic inden-1-ol with vinyl acetate fail whereas the kinetic deacetylation of (I) proceeds in high yield and enantioselectivity. The usefulness of such material is shown by the transformation of (S)-(I) into the optically active amino alcohol (VIII), which is a key starting material for the synthesis of HIV-1 protease inhibitors. The latter transformation is also a determination of the absolute configuration of (S)-(I).