ChemInform Abstract: Remote Electronic Control in Asymmetric Cyclopropanation with Chiral Ru-Pybox Catalysts.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
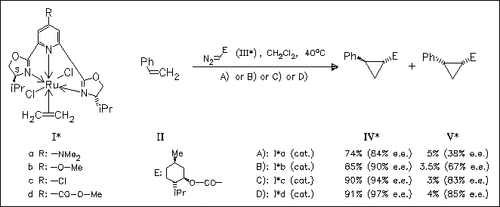
Electronic control by remote substituents far from a catalytically active center is found in an asym. cyclopropanation of styrene (II) and 1-menthyl (III) or ethyl diazoacetates with the Ru catalysts (I*) of chiral 4-substituted bis(4′-isopropyloxazolinyl)pyridine. Electron- withdrawing groups (R: -Cl, -COOMe) increase the catalytic activity as well as the enantiomeric excesses, but electron-donating groups (R: - NMe2, -OMe) decrease them. The enantiomeric ratios of the 1-menthyl and ethyl 2-phenylcyclopropane carboxylates are correlated toward Hammett′s σpara values. However, the trans:cis ratios of the products (ca. 96:4 from (III) and 90:10 from ethyl diazoacetate) are not affected by the substituents R. Intramolecular cyclopropanation clearly gives a similar trend.