ChemInform Abstract: Structure-Based Design of Nonpeptidic HIV Protease Inhibitors from a Cyclooctylpyranone Lead Structure.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
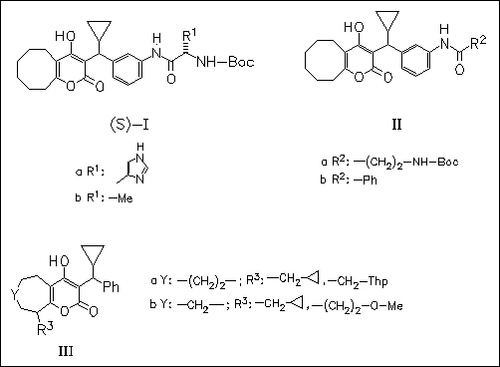
Derivatives of the parent compounds 3-(1-phenylpropyl)- and 3-( cyclopropylphenylmethyl)-4-hydroxy-5,6,7,8,9,10-hexahydrocycloocta(b) pyran-2-one, substituted at the meta position of the aryl ring of type (I) and (II) or at the cyclooctyl ring of type (III) are studied in regard to an enhanced enzyme inhibitory activity. The substitution at the meta position of the aryl ring is far more successful (e.g. ((S)- Ia): Ki=3.0.+-.0.4 nM; ((S)-Ib): Ki=4.0.+-.0.8 nM). An X-ray crystal structure of (IIa) complexed with HIV-1 protease indicated the increase in binding affinity is most likely due to the additional interactions between the amide substituent and the S3 region of the protease.