ChemInform Abstract: Prostaglandin Chemistry. Part 43. Studies on the Preparation of Isocarbacyclin Intermediates. Part 2.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
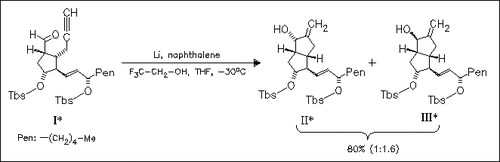
2,2,2-Trifluoroethanol is found to be the most effective proton source for the reductive cyclization of the aldehyde (I) with lithium naphthalene leading to the precursors (II) and (III) of the prostacyclin mimic isocarbacyclin.