ChemInform Abstract: Synthesis of cis-δ-Phenylmethyl-D-proline Using a Nitrogen- Centered Radical Derived from a Chiral Oxaziridine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
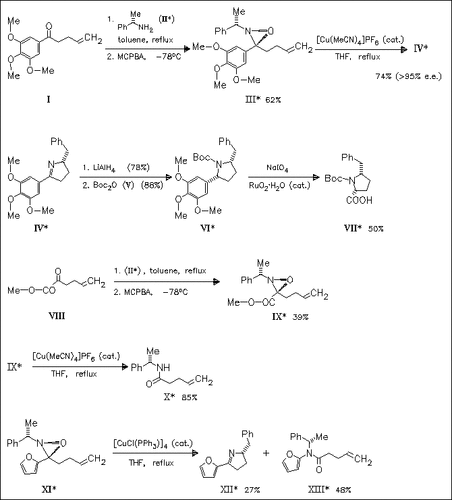
With the interest to unnatural amino acids for designing peptides with unusual conformational and biological properties the title compound ( VII) is synthesized. The key step in this approach is a radical reaction of the oxaziridine (III) to the chiral pyrroline (IV). The cis stereochemistry of (VII) is confirmed by X-ray analysis of the precursor (VI). It is noteworthy that the direct approach starting with the ester (VIII) fails. In the case of the furan derivative (XI) the radical reaction leads to the unexpected product (XIII) in high yield compared to the yield of the desired product (XII).