ChemInform Abstract: Syntheses of Conformationally Restricted Analogues of an Angiotensin II Receptor Antagonist. General Synthetic Approach to Functionalized Imidazo(1,5-a)pyridine Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
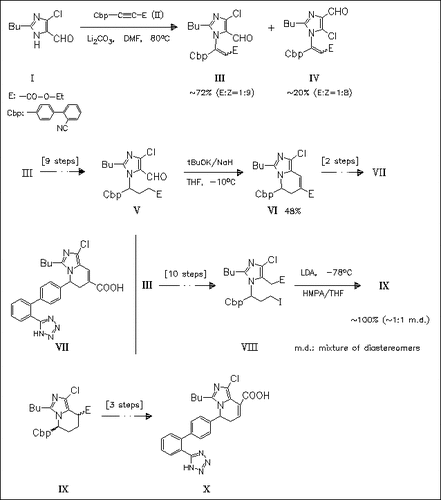
Michael addition of the imidazole-5-carboxaldehyde (I) to the biphenylpropiolate (II) provides an efficient entry to the construction of the skeleton of imidazo(1,5-a)pyridine. By this way the conformationally restricted analogues (VII) and (X) of the nonpeptide angiotensin II receptor antagonist losartan can be prepared. The IC50′s of (VII) and (X) are found to be similar to that of losartan.