ChemInform Abstract: Addition of Organometallic Reagents to N-Glycosyl Nitrones. Enantioselective Syntheses of (+)-(R)- and (-)-(S)-Zileuton.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
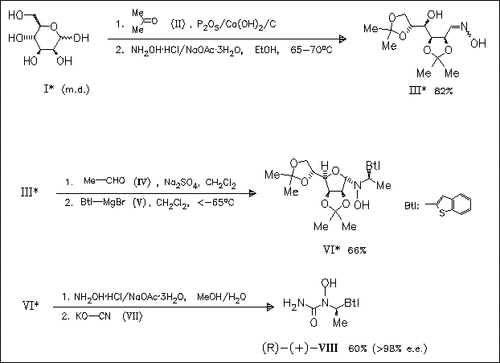
The enantiomers of the 5-lipoxygenase inhibitor zileuton (VIII) are synthesized via the common intermediate (III) by diastereoselective addition of organometallic reagents to the mannose-derived nitrones as key step.