ChemInform Abstract: Monocarboxylates of 3-Methylidene-β-lactams: Synthesis and Unusual Oxidative Rearrangement into a Spiro(azetidine-3,3′- pyrrolidine) Derivative.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
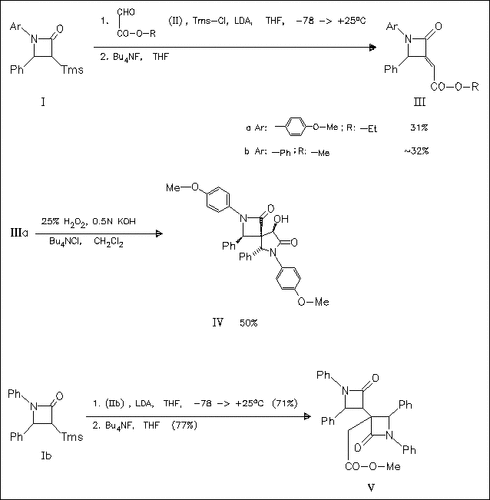
Coupling of the lactams (I) with the glyoxalates (II) in the presence of TmsCl followed by desilylation yields title compounds like (III). Treatment of the latter with oxidizing reagents does not give the expected oxiranes but the spiro compound (IV), whose structure is confirmed by X-ray analysis. In addition, it is shown that the coupling reaction in the absence of TmsCl provides the bis-lactam (V) after desilylation.