ChemInform Abstract: Studies on the Wittig Reaction. Part 15. A Direct Preparation of . omega.-Unsaturated Bromides via Solid/Liquid Transferred Wittig Reactions of ω-Bromoalkyltriphenyl Phosphonium Salts with Aldehydes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
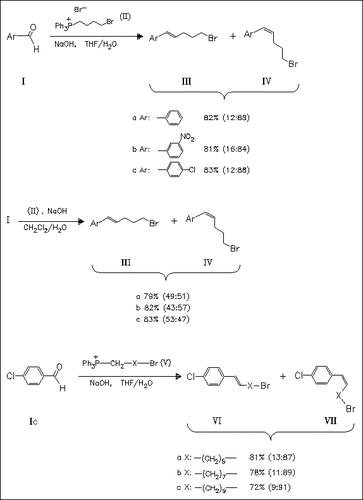
Using solid/liquid transferred Wittig-reaction the title ω- unsaturated bromides (III) and (IV) are prepared in good yields. When THF is used as the solvent, the reaction is (Z)-selective. However, with CH2Cl2 the ratio between (E)- and (Z)-alkenes is approximately 1. In addition, several phosphonium salts differing in the chain length are used. The longer the chain, the lower are the yields, but higher the (Z)-selectivity.