ChemInform Abstract: Asymmetric Synthesis. Part 34. Synthesis of Spiro-Piperidine Derivatives via the CN(R,S) Method.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
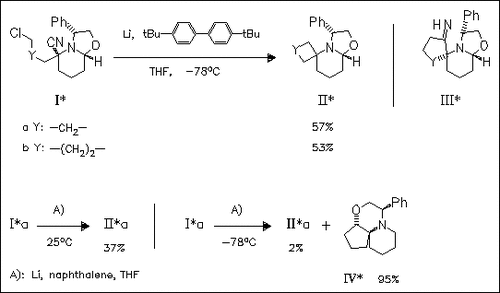
Cyclization of the halogenated cyano-piperidines (I) with lithium di- tert-butylbiphenyl does not give the expected imines (III), required as cephalotaxine moieties, but the spiro derivatives (II) by reductive decyanation. Reaction of (Ia) with lithium naphthalide depends on the reaction temperature. Product (IV) is proved to be formed from intermediate (IIIa).