ChemInform Abstract: Titanium(III)-Induced Transformation of Hydroxylamines to Imines or Secondary Amines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
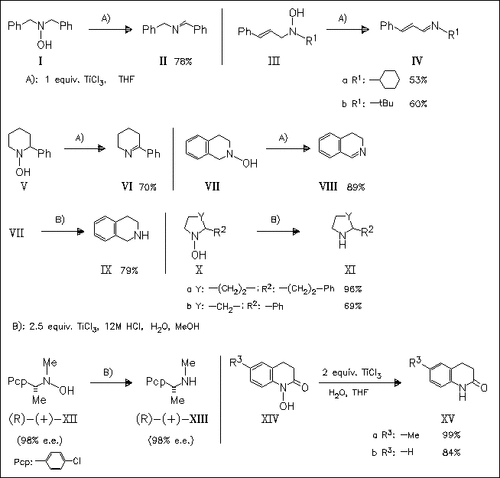
N,N-Disubstituted and cyclic hydroxylamines can be converted efficiently into the corresponding imines on treatment with anhydrous TiCl3. On the other hand, the same hydroxylamines can be converted into the corresponding sec. amines on treatment with aqueous TiCl3. Likewise optically active hydroxylamines which have a chirality at the α-position to the N atom can be converted into optically active sec. amines without loss of chirality. The present TiCl3-mediated reduction can be applied to the reduction of cyclic hydroxamic acids to lactams (e.g. (XIV) → (XV)).