ChemInform Abstract: Copper(I)-Promoted Allylic Nucleophilic Substitutions: A Synthetic and Mechanistic Study.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
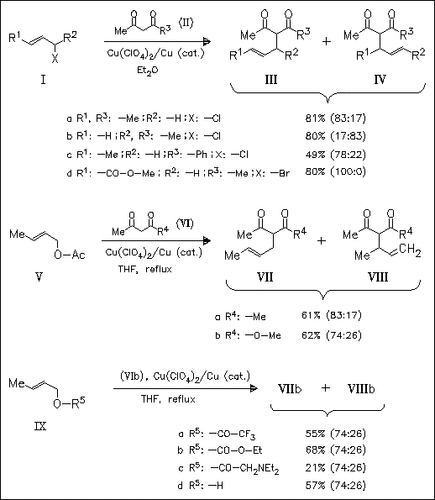
Copper(I) can promote allylic substitutions under acidic conditions. A wide variety of allylic substrates, e.g. (I), (V), and (IX) with different leaving groups are studied in order to investigate the mechanism. The driving force for the reaction is the exchange of the poorly coordinating anion on the copper for a good ligating anion from the allylic substrate. The reaction proceeds through a highly reactive, symmetrical intermediate. Unlike the Pd catalyzed reactions the intermediacy of an η3 allylic complex is not necessary.