ChemInform Abstract: Stereoselective Addition of Organoaluminum or Organomanganese Reagents to α-Formyl Amides or α-Methyl-Substituted β-Keto Amides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
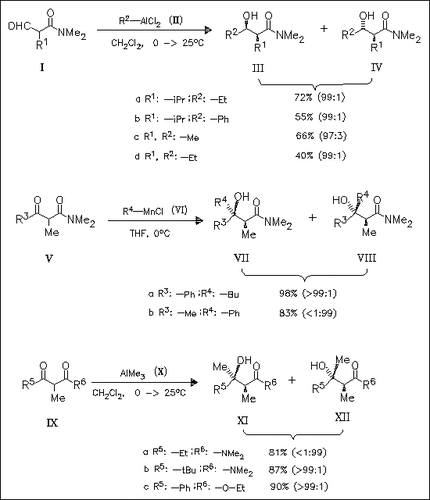
Treatment of the α-formyl amides (I) with alkyl- or phenylaluminum dichloride such as (II) provides threo-α-alkyl- substituted β-hydroxy amides of type (III) under high stereocontrol. This method is successfully applied to the selective addition of an alkyl or a phenyl group to α-methyl-substituted . beta.-keto amides or esters of type (V) and (IX) using alkyl- or phenylmanganese chloride, e.g. (VI), or trialkylaluminum such as (X).