ChemInform Abstract: Synthesis of DD-Heptose Phosphates as Substrates or Potential Inhibitors for the Heptose Synthetase.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
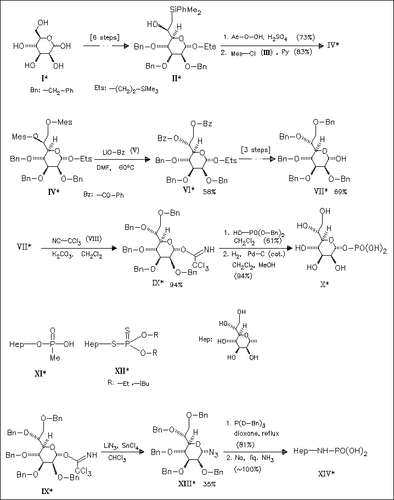
A synthesis of D-glycero-D-manno-heptose compounds (VI), (VII), (IX) via an inversion of L-glycero-D-manno-heptose ((IV) → (VI)) is described. Starting with the trichloroacetimidate (IX), a very successful glycosyl donor in the reaction with phosphate esters, the . alpha.-1-phosphate (X) and the α-1-dialkyldithiophosphate esters (XII) are synthesized. The α-1-methylphosphonate (XI) can be obtained by reaction of (VII) with MePOCl2 followed by Na/NH3 treatment. Amidophosphates, e.g. (XIV), are prepared from (IX) according to the Staudinger reaction using glycosyl azides such as ( XIII) and phosphites. The modified phosphates represent potential inhibitors for the heptose synthetase, an enzyme of the biosynthesis of the inner core region of lipopolysaccharides.