ChemInform Abstract: Reaction of the Lithium Derivative of Diethyl 2-(or 3-) Methylphenylmethanephosphonate with Ketones. An Example of High Syn- Stereoselectivity.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
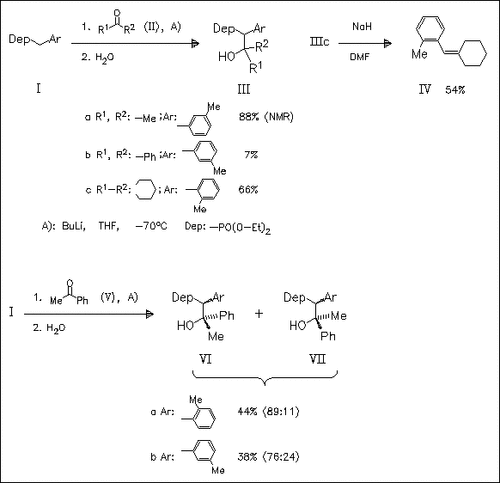
The reaction of the Li derivatives of the arylmethanephosphonates (I) with a large number of sym. and unsym. ketones, e.g. (II) and (V), is studied. The corresponding adducts such as (III) and diastereomeric mixtures (VI) and (VII) with prevailing “threo” isomer are isolated. The results of stereospecific olefination of some β- hydroxyphosphonates, e.g. (IIIc) → (IV), indicate the influence of combined steric effects in ketones and ortho- and meta-substituted benzylphosphonates (I). Spectral investigations and PM3-calculations prove high synstereoselectivity of the reaction of ortho-methyl- substituted benzylphosphonates with the studied ketones.