ChemInform Abstract: An Unprecedented Cleavage of the β-Lactam Ring: Stereoselective Synthesis of Chiral β-Amido Cyanides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
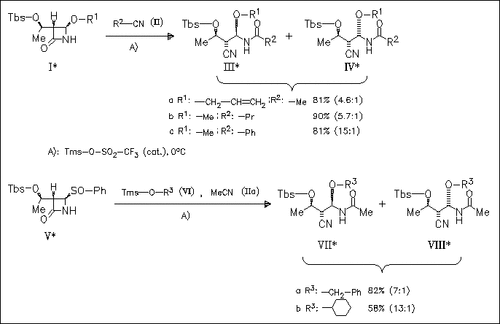
A new method is developed for the synthesis of chiral β-amido cyanides of types (III) and (IV) as well as (VII) and (VIII) by means of the regioselective cleavage of β-lactams of types (I) and (V).