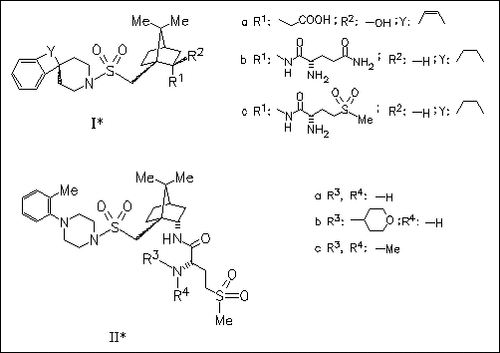
ChemInform Abstract: 1-(((7,7-Dimethyl-2(S)-(2(S)-amino-4-(methylsulfonyl)butyramido) bicyclo(2.2.1)heptan-1(S)-yl)methyl)sulfonyl)-4-(2-methylphenyl) piperazine (L-368,899): An Orally Bioavailable, Non-Peptide Oxytocin Antagonist with Potential Utility for Managing Preterm Labor.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Modification of the selective, orally active oxytocin (OT) antagonist ( Ia) by introduction of the glutaminylamide moiety as in (Ib) improves the OT receptor affinity. Replacing the carboxamide group with the methylsulfonyl group as in (Ic) results in improved potency for antagonizing OT-induced uterine contractions in vivo by both intravenous and intraduodenal routes of administration. Further enhancement of in vivo potency is achieved by transformation into the tolylpiperazine analogue (IIa). Although measurable increases in OT receptor affinity and in vivo potency are produced by derivatization to (IIb) and (IIc), title compound (IIa) exhibits the best overall profile of OT receptor affinity, potency for inhibition of OT- stimulated contractions of the isolated rat uterus and in situ uterus, aqueous solubility and oral bioavailability.