ChemInform Abstract: Multi-Step Redox Systems. Part 61. Synthesis of 1,4-Bis(2-methyl- indolizin-1-yl-methyl)-benzene and Related Model Compounds.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
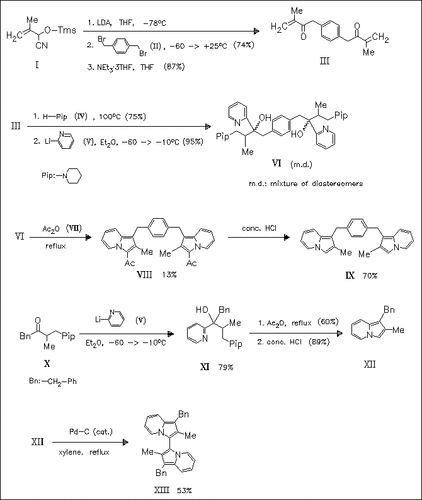
The title compound (IX) is synthesized via its 3,3′-bis-(acetyl) derivative (VIII) by ring closure reaction of the precursor (VI) in refluxing acetic anhydride. This route is first tested by the synthesis of the benzyl indolizine (XII). The latter can be oxidatively dimerized to the 3,3′-bis(indolizine) (XIII) which forms a perfectly reversible two step redox system with KSEM = 4.7×105.