ChemInform Abstract: Synthesis of Naphthoquinone Derivatives. Part 13. A Novel Synthesis of Naphthazarin and Juglone Derivatives by Reaction of Naphthazarin and Juglone with Cyclic Enol Ethers.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
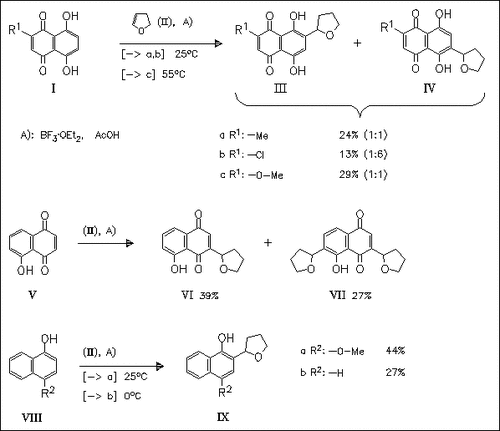
A new method to introduce a side chain into the benzene or quinone ring of naphthazarins, juglone as well as naphthols is described. The reaction with cyclic enol ethers such as (II) in the presence of BF3. times.Et2O gives derivatives like (III), (IV), (VI), (VII) and (IX).