ChemInform Abstract: Enantiospecific Synthesis of All Four Diastereomers of 2-Methyl-3-(( trialkylsilyl)oxy)alkanals: Facile Preparation of Aldols by Non-Aldol Chemistry.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
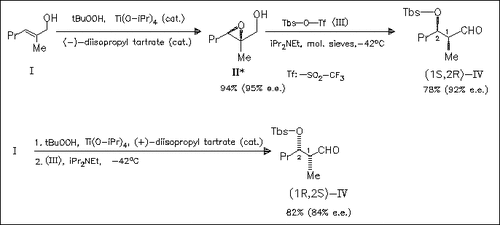
A new method for synthesizing all the four possible diastereomers of the title aldehydes in high yield and excellent enantioselectivity by a unique non-aldol route involving an intramolecular hydride transfer as the key step is given. Whereas (E)-allylic alcohols (cf. (I)) give the syn-aldol products (cf. (IV)) in this facile epoxidation/ rearrangement sequence, (Z)-allylic alcohols furnish the anti-aldol products, respectively. Mechanistic suggestions are given.