ChemInform Abstract: Arylpropanoids from Myristica castaneifolia (Myristicaceae).
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
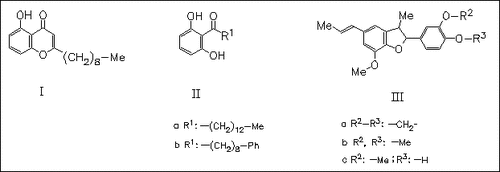
Besides the ketones (II) (isolated previously from other Myristica species) and the neolignans (III) the new benzopyranone (I), named castanone, is isolated from the combined bark and heartwood of Myristica castaneifolia (MC) and its structure is determined by spectral data. The antidiarrhoeal properties of the South Pacific MC may be attributed to the presence of the neolignans (III) with known antibacterial activity. (IIb) undergoes oxidation to a brown pigment, when exposed to air and light. This oxidation product may account for the color turning to dark red, when the heartwood of MC is cut and used as durable timber in the manufacture of furniture.