ChemInform Abstract: Synthesis of 2′-Deoxy-2′-spirocyclopropyl Cytidine as Potential Inhibitor of Ribonucleotide Diphosphate Reductase.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
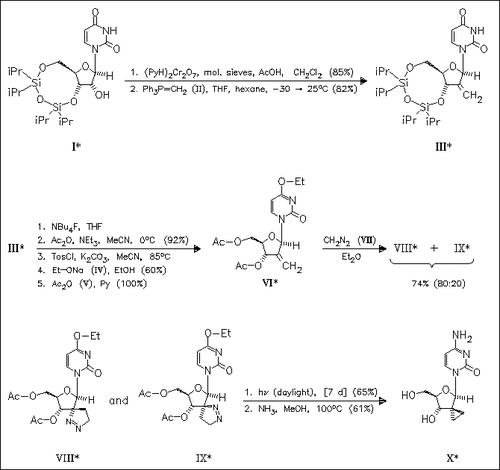
Ribonucleoside diphosphate reductase appears to be a very attractive target for new anticancer agents and research has concentrated on a better understanding of its mechanism of action and the design of selective inhibitors. Based on the mechanism of action of the enzyme, a new class of nucleosides, the title compound (X), which may act as inhibitors is designed. The key step of the synthesis is the condensation with diazomethane giving a (separable) mixture of (VIII) and (IX).