ChemInform Abstract: Tetrathiafulvalene Quinones, Hydroquinones and Esters.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
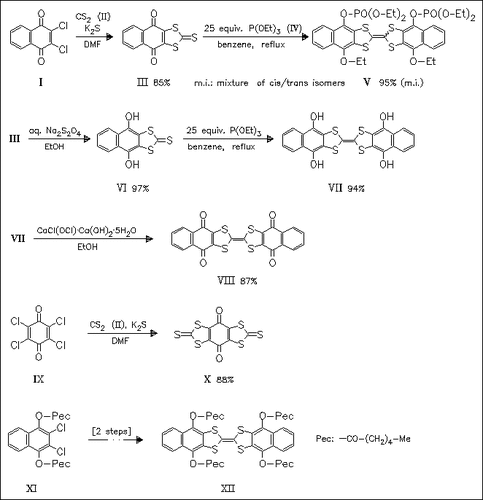
The title tetrathiafulvalene derivatives, e.g. (VII), (VIII), and (XII) , are prepared in view of their potential use for charge-transfer complexes and radical cation salts. The structures of (III) and cis-(V) are confirmed by X-ray analysis (space group P1 with Z = 2 and P21/c with Z = 4, respectively). Attempted reduction and coupling of (X) with P(OEt)3 produces only a polymeric material. Tetrathiafulvalene quinones possess reducing properties like quinones but do not exhibit the oxidation properties of tetrathiafulvalenes as is shown by cyclic voltammetric studies.