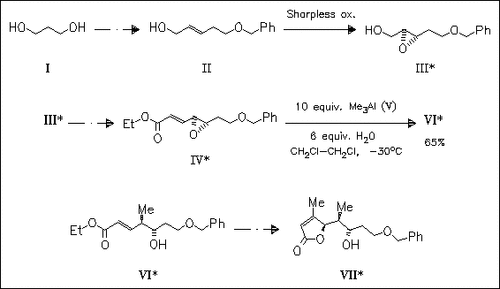
ChemInform Abstract: Stereoconvergent Synthesis of C-9 to C-16 Fragment of Trienomycin Based on the Regioselective Opening of γ,δ-Epoxy Acrylates with Trimethylaluminum.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The chiral epoxide (III), obtained by Sharpless epoxidation of the hydroxyalkenyl benzyl ether (II) is converted to the unsaturated epoxy ester (IV). Its stereoselective cleavage with trimethylaluminum (V), yielding the alcohol (VI), is the key step in the synthesis of the trienomycin precursor (VII).