ChemInform Abstract: Diels-Alder Reactions with an α,β-Unsaturated Aldehyo Sugar. A Route to 6-Oxabicyclo(3.2.1)octanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
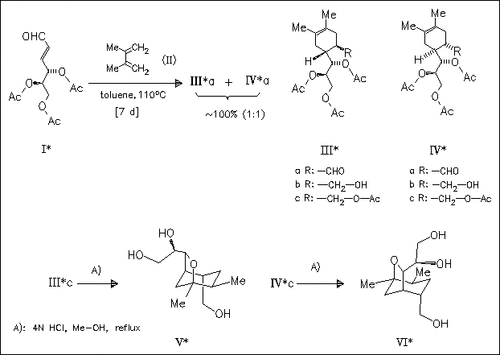
Diels-Alder reaction of the sugar (I) with the diene (II) produces a mixture of the cyclohexenes (IIIa) and (IVa). The derivatives (IIIc) and (IVc), obtained via reduction and acetylation, give the bridged title systems (V) and (VI) (yields not given) upon acidic treatment. The method represents a short approach to this class of compounds in a stereocontrolled manner.