ChemInform Abstract: trans-Dioxides of Cyclic Ketene Thioacetals: Highly Selective Chiral Ketene Equivalents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
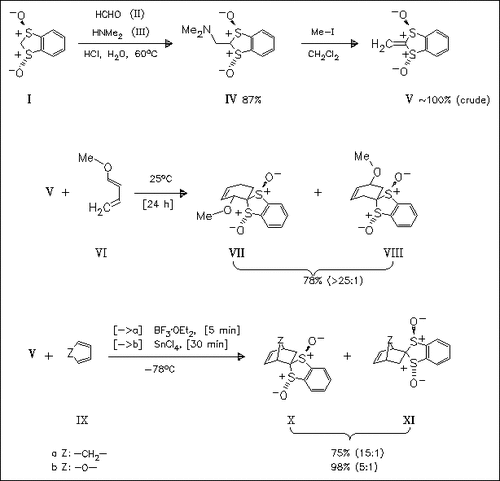
The benzodithiolane dioxide (I) is aminomethylated in an acidic aqueous solution to give the dimethylaminomethyl derivative (IV). In ethanolic solutions, the bis(aminomethylated) analog is formed exclusively. Hofmann degradation of (IV) yields the methylene derivative (V) which represents a ketene dithioacetal S,S-dioxide. (V) is subjected to cycloaddition reactions with various dienes, e.g. (VI) or (IX), to produce the corresponding Diels-Alder cycloadducts with satisfactory diastereoselectivities.