ChemInform Abstract: Transannular Diels-Alder Cyclization of a Substituted 13-Membered Macrocyclic Triene. An Approach to the A.B.C.(6.6.5) Rings of the Veratrum Alkaloids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
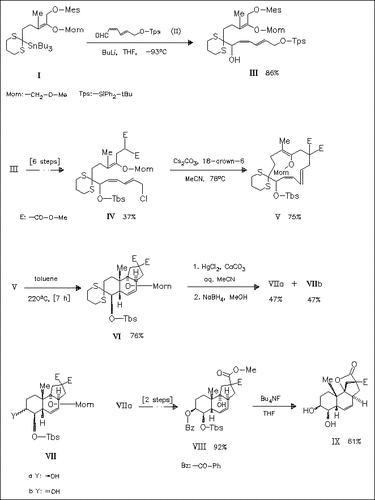
Transannular Diels-Alder cyclization of the 13-membered macrocyclic triene (V) gives the tricycle (VI). Its ring junctions of cis-anti-cis stereochemistry are used to investigate the possibility of introducing a hemiketal oxygen bridge between positions 9 and 4 of the Veratrum alkaloid skeleton. However, only the diol lactone (IX) and its β- benzoate analogue are obtained.