ChemInform Abstract: Convergent Synthesis of (.+-.)-Dihydroisocodeine in 11 Steps by the Tandem Radical Cyclization Strategy. A Formal Total Synthesis of (.+-.) -Morphine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
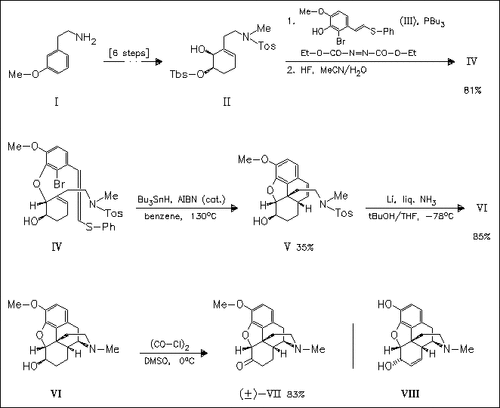
The synthesis of (.+-.)-dihydroisocodeine (VII), a precursor of morphine (VIII), proceeds via tandem cyclization of the aryl ether (IV) to give the tetracyclic styrene (V). The final bond connection is achieved by desulfonation of (V) which results in an unprecedented cyclization to afford the morphine ring system (VI) directly.