ChemInform Abstract: Stereospecific 1,2-Silyl Shift in a Cationic Rearrangement with Retention of Configuration at the Migration Origin.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
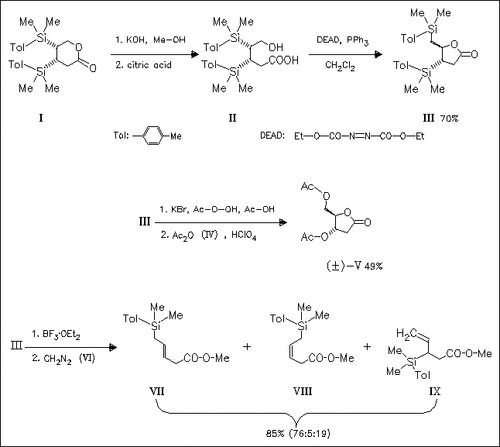
Mitsunobu reaction of the hydroxy acid (II) stereospecifically gives the lactone (III) with retention of the configuration at the origin of migration. This stereochemical course stands in contrast to Hudrlik′s observation that lactones with an embedded silylethyl carboxylate group sometimes undergo acid-catalyzed rearrangement with inversion of the configuration. Treatment of (III) with BF3×OEt3 causes mainly endocyclic elimination (→ (VII)) contradictory to Baldwin′s rule, suggesting that thus elimination follows an E1 pathway.