ChemInform Abstract: Asymmetric Synthesis of 2,6-Diaminopimelic Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
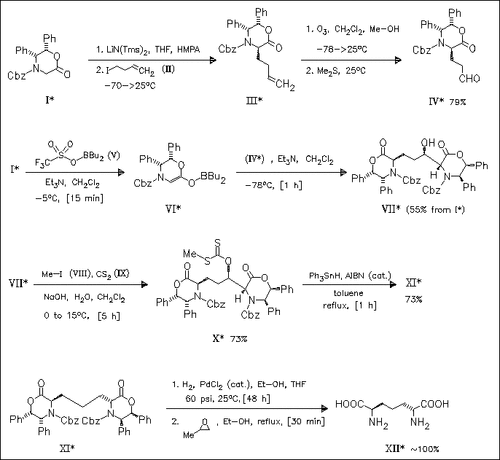
The commercially available chiral lactone (I) is converted to the aldehyde (IV) via the homoallyl derivative (III). (IV) is coupled with the boron enolate (VI) of (I) to give the β-hydroxy bislactone ( VII). Further transformation into the xanthogenate (X) and subsequent triphenylstannane-mediated deoxygenation produce the bislactone (XI) which is subjected to catalytic hydrogenation, yielding (R,R)-2,6- diaminopimelic acid (XII).