ChemInform Abstract: Regioselectivity in the Alkylation of Ambident 2-Pyrimidinone Anions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
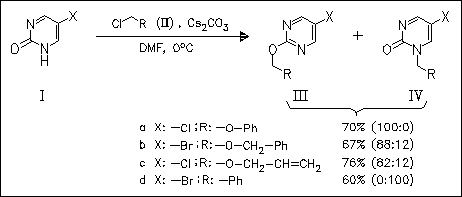
A high selectivity for O-alkylation is observed with hard electrophiles using cesium carbonate as the base in DMF. Reducing the hardness of the electrophile leads to increased alkylation of the softer part of the ambidentate anion, i.e., the N-atom. The halogen substituent in the pyrimidine ring does not seem to influence the yield or product distribution. The comparison of Cs2CO3 to other carbonates of the alkali metals Na, K and Rb shows a great difference. The effect of the cesium cation is explained by its large polarizability hence soft character.