ChemInform Abstract: A New Route to Synthesize 2,9-Dicarbonitrile-1,10-phenanthroline.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
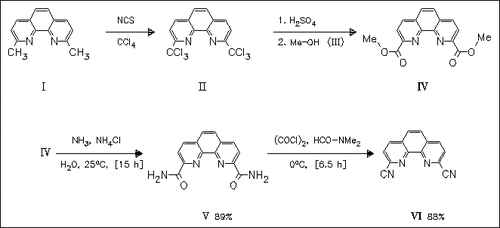
2,9-Dimethyl-1,10-phenanthroline (I) is chlorinated and then esterified to give the dicarboxylate (IV). Treatment of (IV) with concentrated ammonia gives the diamide (V). This is dehydrated to form the dicarbonitrile (VI) by the Vilsmeyer reagent generated in situ from DMF and oxalyl chloride.