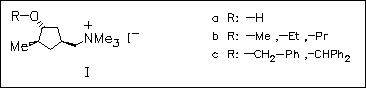ChemInform Abstract: Effect of Deoxamuscarine Etherification on M2 and M3 Muscarinic Affinity and Efficacy.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
In view of the interest in structure-activity relationship of different receptor subtypes, a series of known and newly synthesized ( e.g. (Ic)) ether derivatives of deoxamuscarine (Ia) are tested for muscarinic activity on guinea-pig heart, ileum, and bladder. The results show that introduction of the benzyl group generates an agonist which is 5-fold more potent and 10-fold more selective than ( Ia) on ileum. However, replacement of the hydrogen atom of the hydroxy group by a larger group effects affinity and efficacy differently. The results are used as basis for designing new functionalized congeners for preparing more potent and selective muscarinic ligands.