ChemInform Abstract: Aromatic Sesquiterpenes. Part 13. Synthesis of Lacinilene A and Its Structural Isomer.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
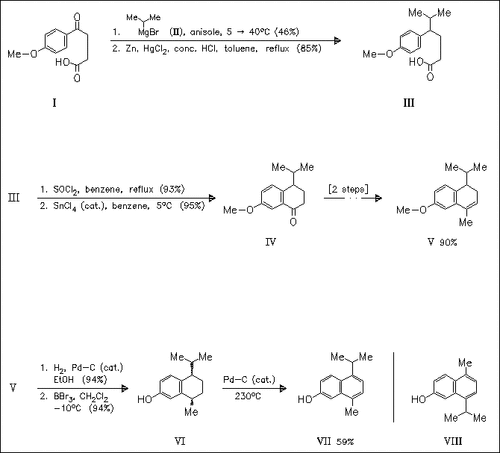
The title norsesquiterpenoid (VII) is prepared from the acid (I) via two alkylation steps and a stereoselective hydrogenation. Grignard methodology is also used for the second alkylation. The synthesis of the title isomer (VIII) proceeds similarly. However, the alkylating agents are used in reverse order and the hydrogenation is less stereoselective. The NMR data of (VII) are identical with those of natural lacinilene A while the data of (VIII) are different.