ChemInform Abstract: 1-Haloalkyl Aryl Sulfoxides as Useful Agents in Synthesis of α- Halo Ketones: A New Synthesis of α-Halo Ketones, α-Halo . alpha.,β-Unsaturated Ketones, and α-Halo Cross Dienones from Aldehydes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
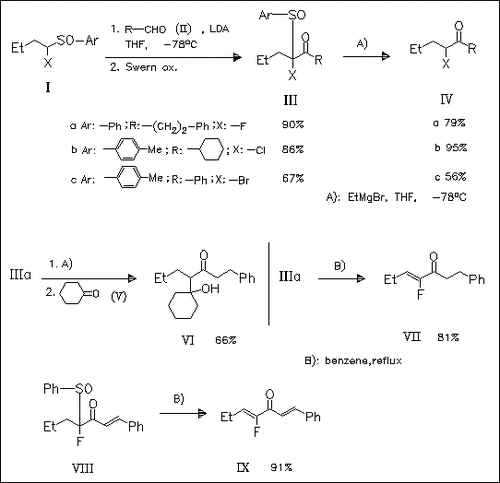
α-Halo α-sulfinyl ketones of type (III) are synthesized in two steps. Their desulfination is achieved with EtMgBr at low temp. to afford α-halo ketones such as (IV). The Mg enolate intermediates can be trapped with various electrophiles (D2O, ClCOOEt, SiMe3Cl). Trapping with ketones (cf. (V)) or aldehydes gives a new type of direct aldol reaction. Thermal elimination of the sulfinyl group under conditions B) yields α-halo α,β-unsaturated ketones or α-halo cross dienones.