ChemInform Abstract: Cycloaddition of 3-Amino-2H-1,4-oxazin-2-ones with Olefins: Generation of 5,6-Dihydro-2-oxo-2H-pyran-6-carbonitriles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
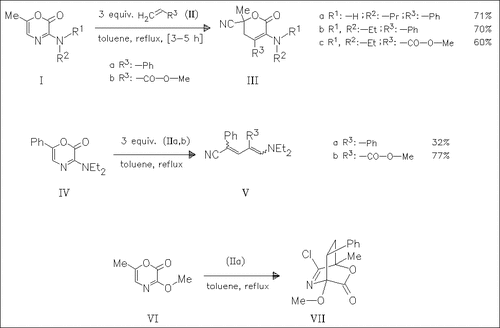
The 1,4-oxazinones (I), (IV), and (VI) are subjected to cycloaddition reaction with styrene (IIa) or methyl acrylate (IIb). Only the 3- methoxy 6-methyl derivative (VI) gives the expected bicyclic primary cycloadduct (VII), whereas the 3-amino analogues (I) undergo subsequent ring transformations, forming the oxopyrancarbonitriles ( III). The 3-amino-6-phenyloxazinone (IV) reacts with the olefinic compounds (II) to produce the aminopentadienenitriles (V) via decarboxylation of the intermediate oxopyrancarbonitriles.