ChemInform Abstract: Synthesis of 3,4-Bridged Indoles by Photocyclization Reactions. Part 1. Photocyclization of Halogenoacetyl Tryptophan Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
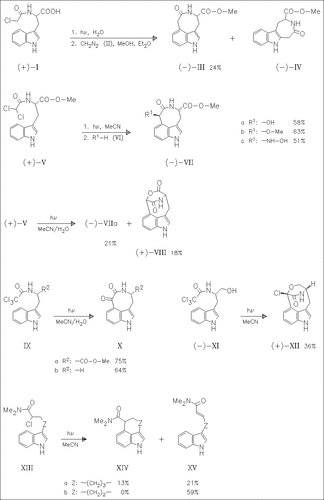
Irradiation of the chloro amide (I) followed by methylation leads to the fused indoles (III) and (IV), while photolysis of the dichloride ( V) and subsequent treatment with the nucleophiles (VI) yields 3,4- bridged indoles exclusively. In the case of the reaction in MeCN/H2O solution, the alcohol (VIIa) and the lactone (VIII) which results from spontaneous cyclization of the cis isomer of (VIIa) are obtained. Irradiation of the trichlorides (IX) in MeCN/H2O also gives the desired 3,4-bridged indoles, while the analogue (XI) is transformed into the cyclic ether (XII). Starting from the chloro amides (XIII) the fused indoles (XIV) and/or the elimination products (XV) are formed.