ChemInform Abstract: Cyanomethyl Esters: Useful Protection for Carboxylic Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
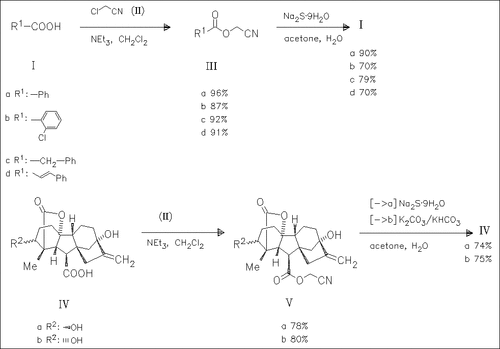
Carboxylic acid cyanomethyl esters are hydrolyzed under very mildly basic conditions using Na2S or a K2CO3/KHCO3 mixture in aqueous acetone. As the cyanomethyl esters can be prepared from the carboxylic acids without prior activation, their formation and subsequent cleavage represent an effective method for the protection of even sterically hindered carboxylic groups in acid-sensitive compounds such as gibberellic acid (IVa) and 3-epi-gibberellic acid (IVb).