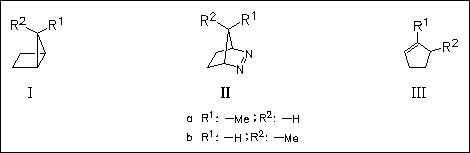ChemInform Abstract: Photochemical and ESR Spectral Evidence for a Stereoselective Rearrangement of Radical Cations Derived from Azoalkanes and Bicyclopentanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Studies of photosensitized electron transfer reactions of (I) and (II) reveal a remarkable stereochemical memory effect. They undergo interconversion and rearrangement into cyclopentenes (III). However, ( Ia) and (IIa) afford only (IIIa) as the rearrangement product, while in the case of (Ib) and (IIb) the rearrangement into the isomeric ( IIIb) is preferred over (IIIa). ESR investigations evidence that the pathway responsible for the stereoselective olefin formation can be assigned to a radical cation rearrangement.