ChemInform Abstract: Electrophilic Amination of C-H Acidic Compounds with 1-Oxa-2-azaspiro( 2.5)octane.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
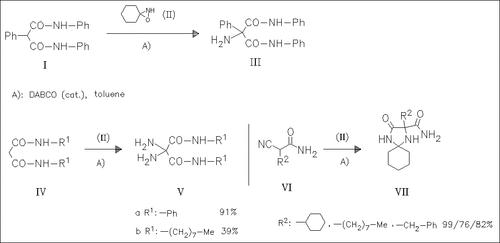
Reactions of the title spirooctane (II) with malonic (cf. (I), (IV)) and cyanoacetic acid derivatives (cf. (VI)) (13 examples in all) are investigated in detail. Several stabilization reactions after the primary amination step (introduction of a 1-hydroxycyclohexylamino group) are observed. Besides simple cyclohexanone eliminations (cf. ( III), (V)), an intramolecular nucleophilic attack to a nitrile group followed by a fast Dimroth rearrangement is one of the most important ones yielding diazaspirodecanones (cf. (VII)).